Insight
Pyxis Discovery gears up for covalent drug discovery race
Covalent-acting drugs prove their case
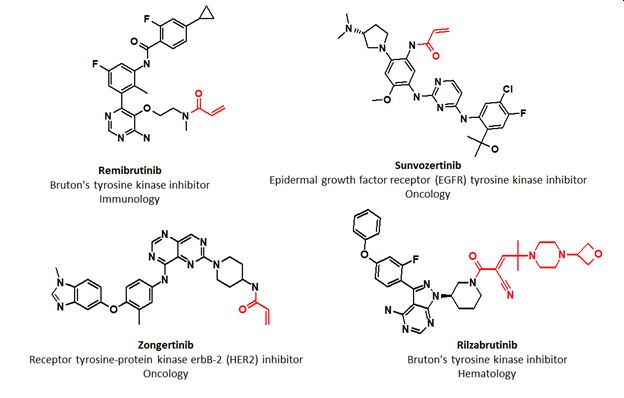
The year 2025 witnessed remarkable achievements in the approval of covalent-acting small molecule drugs by the FDA. By December, four drugs (Remibrutinib, Rilzabritinib, Zongertinib, and Sumvozertinib) had been approved. Each drug incorporated an acrylamide warhead designed for covalent binding to cysteine residues in the corresponding targeted kinases:
Opportunities for complex proteins
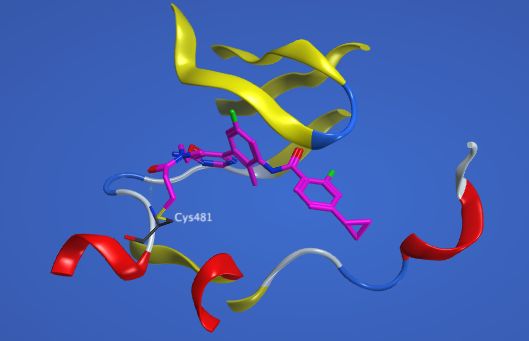
The scope of potential targets for covalent small molecule inhibitors extends well beyond just kinases, creating a distinctive opportunity for macrocycles to play a pivotal role in designing new and effective inhibitors for protein-protein interactions, proteases, GTPases, as well as proteins associated with viruses, malaria, and bacteria:

Driving selectivity and affinity
In the context of covalent inhibitor design, a macrocyclic scaffold plays a key role in the initial molecular recognition within the protein binding site. It achieves this through various non-covalent interactions (hydrogen bonds, hydrophobic interactions, and van der Waals forces). This initial binding is essential for achieving high affinity and selectivity. By enriching macrocyclic scaffolds with diverse elements (e.g. pharmacophoric fragments, heterocyclic rings, linkers, and functional groups) it is possible to enhance overall potency. This also favors orientation of the electrophilic warhead toward a key nucleophilic “hot spot” residue. Pyxis macrocycles are particularly versatile due to their chemical properties. These enable the incorporation of multiple covalent warheads that can target cysteine, lysine, tyrosine, and serine residues.
Delivering efficiency
To validate our platform, we initially established a high-throughput synthesis protocol. It efficiently produces thousands of cysteine-oriented electrophilic macrocycles in just a few days. Given the reactive properties of covalent warheads, we meticulously optimized purification conditions. This ensures the purity and stability of the compounds over time. The resulting library includes thousands of distinct macrocyclic derivatives. It is now accessible for evaluation and screening. For information on accessing the library or to discuss your specific project with our scientific team, please reach out to us.